Ongoing Projects
Our lab aims to reveal general principles involved in neuronal identity and synaptic specificity and advance our understanding of how neural circuits emerge during development. Significantly, our research has the potential to identify novel therapeutic targets for respiratory dysfunction. Most ALS patients die from respiratory failure within 3-5 years of diagnosis and current treatments do not slow disease progression. Developmental disorders, such as Rett Syndrome, also exhibit a profound respiratory component due to defects in respiratory circuitry. The generation of phrenic MNs from embryonic stem cells holds promise as a therapeutic avenue but integrating these MNs in existing circuits has been challenging. Understanding the molecular mechanisms involved in respiratory circuit formation will undoubtedly bring us closer to the development of effective treatment methods for breathing disorders.
What are the molecular mechanisms that govern phrenic motor neuron identity?
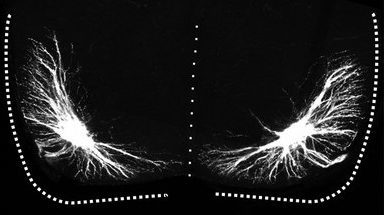
We have demonstrated that Hox5 genes are required for the development of diaphragm-innervating phrenic motor neurons (MNs). Selective deletion of Hox5 genes from MNs in mice leads to perinatal lethality due to breathing defects, an extinction of phrenic MN identity, progressive loss and disorganization of phrenic MNs, axon guidance defects and a dramatic loss in synaptic contacts at the diaphragm muscle. These diverse defects are likely the result of coordinated transcriptional regulation of a cohort of genes by Hox5 proteins. We utilize RNA and chromatin immunoprecipitation sequencing to identify phrenic-specific Hox5 transcriptional targets and use novel gene editing techniques such as CRISPR to assess their role in phrenic MN development in vivo.
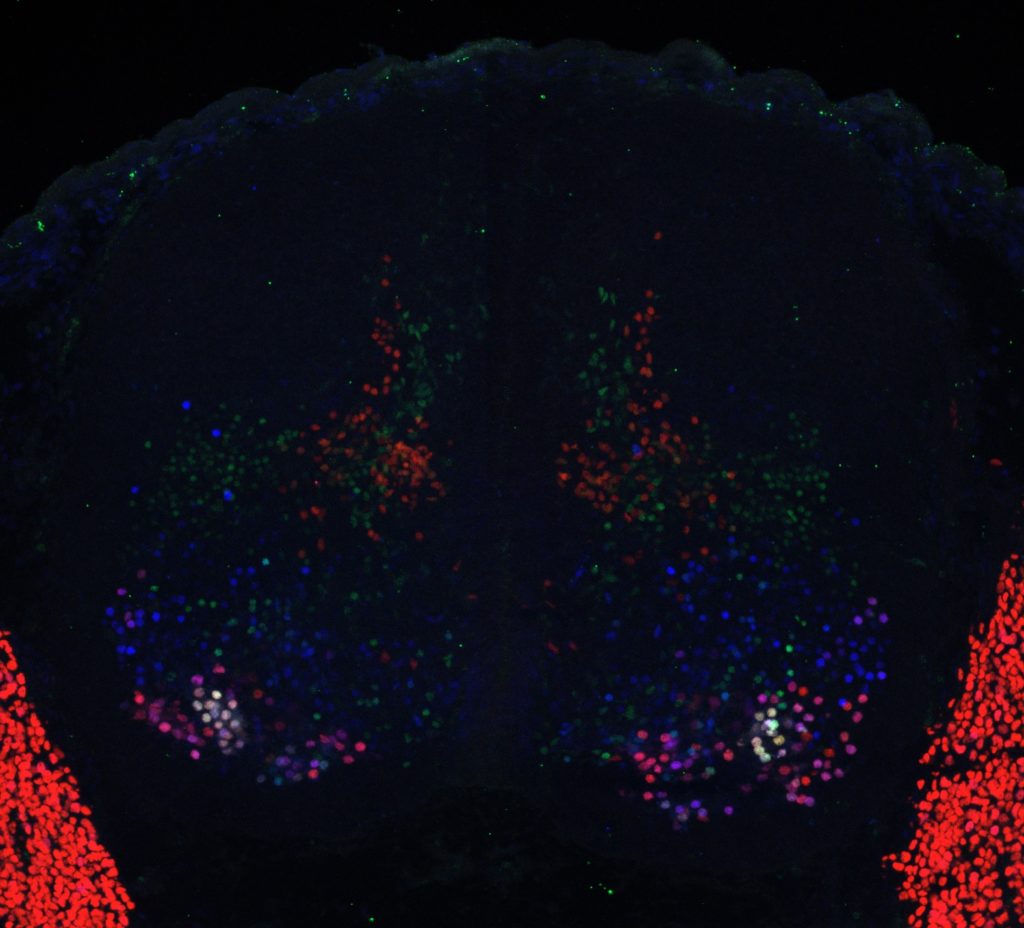
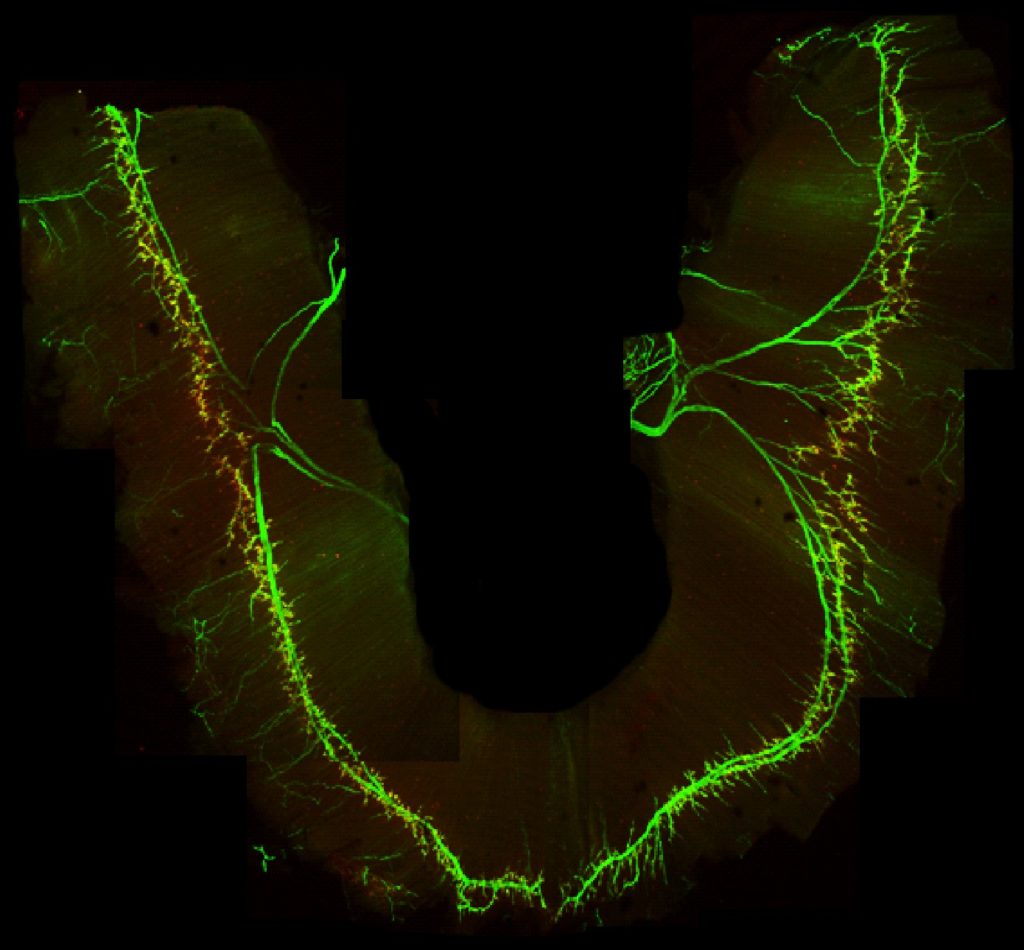
How do transcriptional programs define respiratory circuit assembly?
Functional assembly of neural circuits requires that individual circuit components establish multiple inputs. A prevailing, but poorly tested, hypothesis has been that the acquisition of specific subtype identities through transcriptional programs deploys molecular pathways underlying connectivity. Removal of Hox5 genes from phrenic MNs eradicates their identity, providing a powerful molecular entry point to test this hypothesis. We employ viral tracing methods, electrophysiology and plethysmography to assess how phrenic MN inputs are established during development.
How do extrinsic signals influence phrenic motor neuron connectivity?
The neuronal networks that govern stereotypical behaviors such as breathing and walking are thought to be largely established through hardwired transcriptional programs. Nevertheless, breathing exhibits a remarkable potential for plasticity even before birth. Both gender and gestational stress affect newborn breathing behaviors, likely through rewiring of respiratory circuits, and these changes persist to adulthood. We use a genetic in vivo approach to define how environmental signals intersect with transcriptional programs to establish respiratory circuits.